seliwanoff test principle|Seliwanoff’s Test: Principle, Reagent Preparation, Procedure And : Pilipinas Principle. The principle behind Seliwanoff’s Test lies in the specific reaction between the sugar being tested and a specialized reagent known as Seliwanoff’s reagent. The . Places chargées valables 6 mois à compter de la date d'achat. Dans la limite de 3 places maximum par séance. 8.10 € la place. Chargement 10 places. Soit 76.00 €, frais de gestion inclus. Places chargées valables 12 mois à compter de la date d'achat. Dans la limite de 4 places maximum par séance. 7.60 € la place. Chargement 15 places
seliwanoff test principle,Principle of Seliwanoff’s test. The reagent of this test consists of resorcinol and concentrated HCl. The acid hydrolysis of polysaccharides and oligosaccharides yields simpler sugars. Ketoses are more rapidly dehydrated than aldoses. Ketoses undergo dehydration in the presence of concentrated acid to . Tingnan ang higit pa
Seliwanoff’s test is used to differentiate between sugars that have a ketone group (ketose) and sugars that have an aldehyde group . Tingnan ang higit pa Seliwanoff’s test is a chemical test which differentiates between Aldose and ketose sugars. If the sugar contains a ketone group, it is a ketose whereas if it .Seliwanoff’s test is a chemical test which distinguishes between aldose and ketose sugars. If the sugar contains a ketone group, it is a ketose. If a sugar contains an .Principle. The principle behind Seliwanoff’s Test lies in the specific reaction between the sugar being tested and a specialized reagent known as Seliwanoff’s reagent. The . Seliwanoff’s test is a type of chemical test that used to differentiate between ketose and aldose sugars. If the sugar has a ketone group, then it’s ketose. If . Seliwanoff’s test is a compound test to separate aldose and ketose sugars. It uses HCL, resorcinol and a red reagent to detect the presence of ketones in a solution. Learn how to prepare the reagent, .

Seliwanoff’s test is a chemical test to distinguish between ketoses and aldoses based on their reaction with a reagent composed of resorcinol and hydrochloric acid. . In Seliwanoff’s test, a dehydration reaction is involved. Seliwanoff’s reagent contains a non-oxidizing acid (HCl) and resorcinol. When a ketose (sugars with a ketone . This test employs a timed colour reaction peculiar to ketohexoses. Objectives of Seliwanoff’s test. to evaluate whether or not a sample contains ketohexoses. to separate aldoses from ketoses. .How to perform the test: One half ml of a sample solution is placed in a test tube. Two ml of Seliwanoff's reagent (a solution of resorcinol and HCl) is added. The solution is then . This is the video on Seliwanoff's Test With Demonstration and Discussion of viva questions.0:00 - Introduction00:36 - Principle of Seliwanoff's Test01:05 - P.
The principle behind Seliwanoff’s Test lies in the specific reaction between the sugar being tested and a specialized reagent known as Seliwanoff’s reagent. The reagent itself consists of resorcinol, a colorless organic compound, dissolved in an acidic solution. When heated together with a sugar sample, such as fructose or glucose, the .

Qualitative Test of CarbohydratesSeliwanoff’s testSeliwanoff's test is a chemical test which distinguishes between aldose and ketose sugars. If the sugar con.seliwanoff test principle Seliwanoff’s Test: Principle, Reagent Preparation, Procedure AndProcedure of Seliwanoff’s test. Take two clean, dry test tubes and add 1 ml of the test sample in one test tube and 1 ml of distilled water in another as blank. Add 2 ml of Seliwanoffs’ reagent to both the test tubes. Keep both the test tubes in a water bath for 1 min. Observe the formation of color and note it down.Seliwanoff's test is used to differentiate between aldose and ketose, i.e., it helps to detect the presence of aldo sugar and keto sugar. Seliwanoff's reagent is a mixture of resorcinol and concentrated hydrochloric acid. Seliwanoff’s test with aldose and ketose. Ketose sugars react with the Seliwanoff's reagent to give immediately a deep .Seliwanoff's Test is a test to distinguish keto sugars form aldo sugars. Ketoses form furfurals more rapidly than aldoses. A compasison of the rate of colour formation is the bases of distinguishing between these two types of sugars. At the end of the procedure, an observation of the test result is made and interpreted and a well balanced .
Seliwanoff’s Test. This test is used to detect monosaccharides with a ketonic functional group. It is widely used to differentiate fructose, a keto sugar, from glucose and galactose. Principle. This test involves the formation of furfural derivatives by monosaccharides with hydrochloric acid.
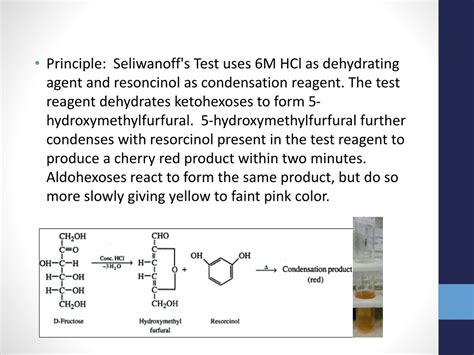
Principle of Bial’s Test. This test is based on the principle that under hydrolysis pentosans are hydrolyzed into pentoses. Further, pentoses are dehydrated to yield furfural, which in turn condense with orcinol to form a blue-green precipitate. In the presence of hexoses, hydroxyfurfural is formed instead of furfural which upon . The original Seliwanoff test, a qualitative color test used for the presence of fructose, was developed by the Russian chemist Theodor Seliwanoff (Roe, 1934; . Based on this reaction principle of the Seliwanoff test, the following research questions were asked: if the determination of fructose is carried out by converting it to HMF using the . In studies, the concentration of resorcinol and HCl acid used for the Seliwanoff reagent varied from 0.03 to 0.1% and 10-18%, respectively, to increase the sensitivity of the test (Amine et al .• Principle: Seliwanoff'sTest uses 6M HCl as dehydrating agent and resoncinolas condensation reagent. The test reagent dehydrates ketohexoses to form 5‐ hydroxymethylfurfural. 5‐hydroxymethylfurfural further condenses with resorcinol present in the test reagent to
seliwanoff test principle Seliwanoff’s test is a biochemical test which is used to identify the presence of ketose and aldose. This test is based on the principle that, on heating or on treatment with a concentrated acid, ketoses dehydrate more rapidly than aldoses. The reagent used in Seliwanoff’s test consists of resorcinol and concentrated hydrochloric acid .Quick Reference. A biochemical test to identify the presence of ketonic sugars, such as fructose, in solution. It was devised by the Russian chemist F. F. Seliwanoff. A few drops of the reagent, consisting of resorcinol crystals dissolved in equal amounts of water and hydrochloric acid, are heated with the test solution and the formation of a . Procedure of Barfoed’s Test. Take 1 ml of a given sample in a clean, dry test tube. The concentration of disaccharides sample (if used) should not exceed 1% (w/v). Take control of 1 ml of distilled water in another tube. Add about 2-3 drops of Barfoed’s reagent to both the tubes and mix them in a vortex.Seliwanoff’s Test: Principle, Reagent Preparation, Procedure AndPrinciple: Keto sugars get dried out in the nearness of concentrated acids to yield furfurals or their subsidiaries which react with resorcinol in Seliwanoff reagent to yield a cherry-red hue complex as the positive result. Procedure: Take a test tube, and add 5 ml of Seliwanoff’s reagent to it.Precautions: Wash the apparatus before and after the experiment. Avoid prolonged heating as it will then gives a false result. Seliwanoff's test: Seliwanoff's test is used to differentiate between keto sugar and aldo sugar. PRINCIPLE: Carbohydrates are converted to . Principle of Seliwanoff’s test. Seliwanoff’s test is used for difference between ketose and aldose sugars. It is for the detection of fructose i.e this test is positive for fructose and negative for other sugar. With the help of this test we can easily distinguish and detect fructose solution. When the saliwanoff’s reagent is mixed with . Barfoed’s test is a biochemical test used to detect monosaccharide (reducing) sugars in solution. The technique was devised by a Swedish physician C. T. Barfoed (1815–1899). Barfoed’s reagent, a mixture of ethanoic (acetic) acid and copper (II) acetate, is added to the test solution and boiled. If any reducing sugars are present a red .
seliwanoff test principle|Seliwanoff’s Test: Principle, Reagent Preparation, Procedure And
PH0 · Seliwanoff’s Test: Principle, Reagent, Procedure & Result
PH1 · Seliwanoff’s Test: Principle, Reagent, Procedure & Result
PH2 · Seliwanoff’s Test: Principle, Reagent Preparation, Procedure And
PH3 · Seliwanoff’s Test: Principle, Reagent Preparation, Procedure And
PH4 · Seliwanoff’s Test: Principle, Reagent Preparation,
PH5 · Seliwanoff’s Test
PH6 · Seliwanoffs Test Principle and Procedure
PH7 · Seliwanoff's test
PH8 · Seliwanoff's Test Principle, Procedure, Result
PH9 · Experiment
PH10 · Carbohydrates